As the cannabis industry evolves from its unregulated days into the era of full legalization, greater emphasis will be placed on analytical testing of commercial products. Unlike the pharmaceutical or food sectors, high-level scientific research on cannabis is scant due to the draconian restrictions on cannabis in the 20th century. In addition to laws restricting the cultivation, sale, and ingestion of Cannabis, research into possible medical uses of the plant was largely forbidden by major institutions and funding sources, leading to a dearth of peer-reviewed cannabis science until recent decades.
The peer-review process is a foundational building block in the scientific method and ensures that published works contain verifiable findings. Over a century of peer-reviewed science has yielded the sprawling international pharmaceutical and food science sectors, and the high degree of quality we expect from these goods reflects the ongoing efforts of the scientific community. In this regard, the cannabis sector is playing from behind; a historical lack of scientific investigations, combined with rapidly expanding legal markets, leads to a huge need for quality analytical testing with little scientific basis for these tests. A concerted effort is needed from the cannabis scientific community to overcome this disadvantage and help the field begin a new chapter of detailed scientific know-how.
Thankfully, findings from the food science and pharmaceutical sectors can help guide cannabis research. One of the most robust market sectors in cannabis are edibles, which includes cannabinoid-infused chocolates, baked goods, gummies, candies, and more. For example, cannabis-infused chocolates alone accounted for $91.8 million in retail sales in 2018 for the combined legal markets of California, Washington, Oregon, and Arizona. [1]
Despite its ubiquity on retail shelves and testing lab benchtops, chocolate is a deceptively difficult product matrix to analyze. The term ‘matrix’ is an analytical chemistry term that refers to elements of a sample that are not of analytical interest; the compounds of interest you are looking to measure are the analytes. For a potency test on cannabis-infused chocolate, the analytes are the cannabinoids (which are of interest because they affect the desired medicinal or physiological effects) and the matrix is chocolate (which is not of interest as it merely is a vehicle to deliver cannabinoids). Put another way, the matrix is the canvas, and the analytes are the paint. We typically go to art museums to appreciate the paint, not the canvas on which it is displayed. In the same way, cannabis testing laboratories care about the cannabinoid potency and not the chocolate in which they are contained.
The separation of analytes of interest from the matrix (i.e., extraction) is at the heart of all analytical chemistry and is one area in which cannabis analytical science lags far behind the analogous fields of pharmaceutical and food analysis. Some matrices are very straightforward for testing purposes: removal of cannabinoid analytes from Cannabis flower, for instance, is as easy as grinding up the matrix (in this case, the plant material) and adding an organic solvent, such as methanol. The cannabinoid analytes are attracted to the solvent and readily go into solution, which makes for a quick and easy extraction.
Other matrices, such as the chemically complex foodstuffs common to edibles, can make for a much more difficult extraction. In the food science sector, chocolate is known as one of the more difficult food matrices to test. [2] This is, in part, due to the molecular components of chocolate, such as high fats, polyphenols, and tannins, which are known to directly interfere with extraction and detection procedures. [3] When the matrix itself becomes a barrier to analyte extraction, it is known as ‘matrix effects’ or ‘matrix interference.’ Essentially, certain molecular interactions between analytes and elements of the matrix are so strong that our normal extraction strategies cannot free them from the matrix, and some analytes become ‘trapped’ within it. Therefore, matrix interference can lead to erroneously low levels of analyte detection, which, in the context of cannabis-infused edibles testing, would yield lower calculated potency values. Keep in mind — the cannabinoids, our analytes of interest, are still present in the sample; they are simply being under-counted due to matrix effects. Although prior studies from the food science sector on the analytical difficulties surrounding chocolate extraction were performed on allergen analytes, the chemical complexity of chocolate suggests that cannabinoid analyte extraction from chocolate may be similarly vexed.
To determine if there is matrix interference from chocolate during potency testing of cannabis-infused chocolate edibles, researchers in Oakland, CA, designed a set of experiments to put this hypothesis to the test. [4] The study utilized stock solutions of five different cannabinoids (∆9-tetrahydrocannabinol (THC), cannabidiol (CBD), cannabinol (CBN), cannabigerol (CBG), cannabidiol dimethylether (CBDD)) at a known concentration in methanol. These specific cannabinoids were chosen as analytes due to their varied chemical structures, as minute structural differences between the cannabinoid analytes could affect the magnitude of matrix interference. Creating stock solutions of a known concentration is experimentally useful because this knowledge lets us calculate the exact amount of cannabinoids present in the test vial.
Next, various quantities (1g, 2g, 3g) of three uninfused chocolate types (milk chocolate, dark chocolate, and cocoa powder) were added to a given volume of a cannabinoid stock solution. If chocolate does not display matrix interference, we would expect to recover nearly all the cannabinoid analytes present, as there is nothing ‘trapping’ the analytes and erroneously lowering the potency. However, if there is matrix interference from chocolate, we would expect to see lower calculated potency values as the amount of chocolate increases: more chocolate means more interference, which means more ‘trapped’ cannabinoid analytes, and thus lower measured potency values. Remember, the lower measured potency values do not mean the cannabinoids are disappearing, or that the sample is somehow less potent. We know exactly how much analyte went into the test vial thanks to our stock solutions; the only variable at play is how much chocolate is present.
uthor
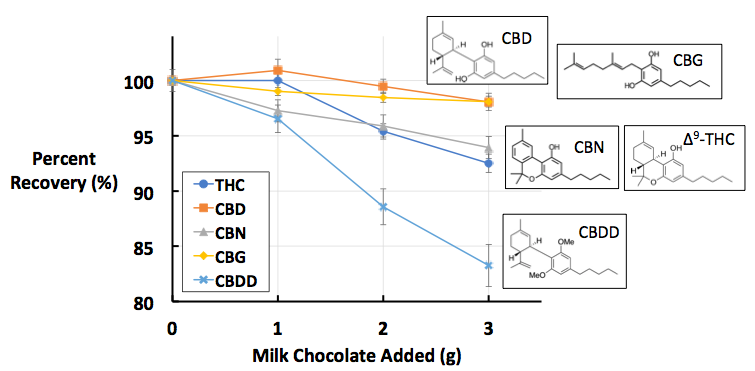
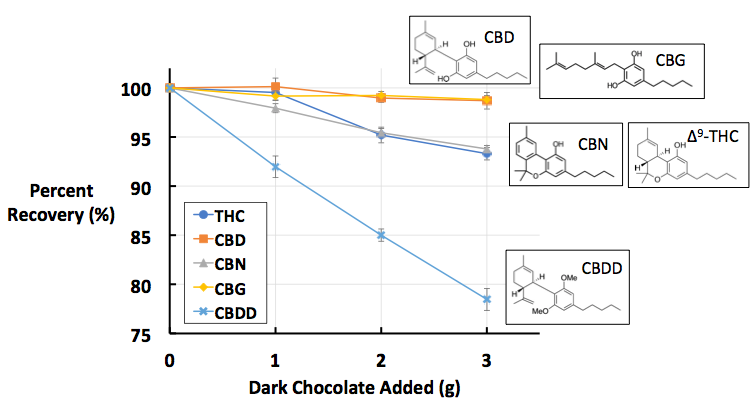
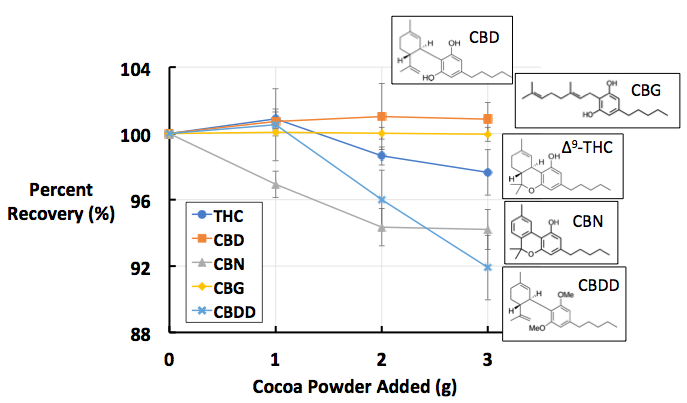
Figure 1: Percent recoveries of five cannabinoids in A) milk chocolate, B) dark chocolate, and C) cocoa powder. All data points are the average of [n = 10] replicates. These images were recreated from the figures published in Dawson D, Martin R. Investigation of chocolate matrix interference on cannabinoid analytes. J. Agric. Food Chem. 2020;68:5699–5706.
The findings of this study can be found in Figure 1. If the findings indicated no matrix interference, we would expect to see the trend lines (each color coded for a specific cannabinoid analyte) as flat, hovering around ~100% recovery. We see something like this with the orange and yellow data sets, which represent CBD and CBG, respectively. This suggests that there is minimal matrix interference between these two analytes and the three types of chocolate tested. This is not the case for the other three analytes.
The dark blue and grey data sets, representing THC and CBN respectively, show a clear downward trend across all three chocolate types: as more chocolate is added, the average percent recovery decreases. This is highly indicative of matrix interference from chocolate on these two analytes. The final analyte, CBDD, represented in light blue, shows the steepest downward trendline, which indicates it suffers from the highest amount of matrix interference. Overall, these findings indicate that not all cannabinoids suffer from the same degree of matrix interference. CBD/CBG has the least, CBDD the most, and THC/CBN in between.
So, why do different cannabinoids suffer from varying degrees of matrix interference? The answer lies in the relatively subtle structural differences between the five tested cannabinoids, as well as the chemical makeup of the chocolates tested. There is a strong correlation between the degree of matrix interference and a certain functional group on the cannabinoid molecule, known as a phenolic –OH group. Phenolic –OH groups are a specific type of alcohol, in which the alcohol’s –OH group is attached to an aromatic six-carbon ring, known as a phenyl group. As seen in Figure 2, there is an inverse correlation between the number of phenolic –OH groups and the amount of matrix interference: the more phenolic –OH groups (e.g., CBD/CBG), the less interference.
The reason for this striking correlation likely has to do with how the phenolic –OH groups affect the polarity of the molecule. Oxygen is a polar atom, so increasing the number of –OH groups on a molecule increases its overall polarity. Conversely, decreasing the number of –OH groups, or eliminating them entirely, will decrease a molecule’s polarity, making it non-polar. The chocolate matrix contains high amounts of naturally occurring fats, which are a class of non-polar molecules (in fact, the chocolate used in this study was over 40% fat by weight). In chemistry, a foundational rule of thumb is that polar molecules are more attracted to polar molecules, just as non-polar molecules are attracted to other non-polar compounds. Mixing polar and non-polar compounds doesn’t work too well, which gives us the idiom ‘like oil and water’ which signifies when two things don’t get along; the fatty, non-polar oil doesn’t mix well with the polar water molecules.
Figure 2: Structures of the five cannabinoid analytes tested, with phenolic –OH groups highlighted in red. These images were recreated from the figures published in Dawson D, Martin R. Investigation of chocolate matrix interference on cannabinoid analytes. J. Agric. Food Chem. 2020;68:5699–5706.
With these chemical factors in mind, we can begin to explain the matrix interference trends seen in Figure 1. CBD and CBG, which both contain two phenolic –OH groups and are thus polar, do not mix well with the fatty, non-polar chocolate matrix. In fact, these two analytes are more attracted to methanol, the polar solvent used as the extraction solvent. This low affinity for the matrix, and high affinity for the extraction solvent, means very little CBD or CBG gets ‘trapped’ in the chocolate matrix, which leads to the high recovery rates seen in Figure 1.
The compounds with one phenolic –OH group, THC and CBN, have more non-polar character, which increases their interactions with the non-polar chocolate matrix. We can see this manifest in their lower recovery rates when compared to CBD and CBG. CBDD, which contains no phenolic –OH groups and thus is the most non-polar analyte tested, is highly attracted to the chocolate matrix, ‘trapping’ a large amount of the cannabinoids in the matrix, leading to lower recovery rates. To a certain extent, the attraction of cannabinoids to fats is unsurprising; numerous cultures have used butters to extract cannabis for medicinal uses [5], and prolonged ingestion of cannabinoids can lead to long-term accumulation in human fatty tissues [6]. However, the finding that minute structural differences on cannabinoids can affect the degree of attraction was unexpected and suggests that there is more nuance to cannabinoid potency testing than meets the eye.
Another nuance can be seen in the data set for CBN in the cocoa powder matrix (Figure 1C). In the milk and dark chocolate plots, CBN and THC behaved extremely similarly to one another, in part due to both analytes containing one phenolic –OH group. However, in the cocoa powder plot, CBN has much lower recovery rates than THC, with lower recoveries than even the highly non-polar CBDD at 1g and 2g. The fact that this outlying data only occurs with the CBN/cocoa powder combination suggests that a different matrix effect could be at play. Cocoa powder differs from milk and dark chocolates in that it contains less fat (25% by weight) and a higher concentration of cocoa solids than finished chocolate products. The chemical makeup of cocoa solids is complex, as the natural extract from the cocoa bean can contain over 70 different organic flavoring molecules. [7] One class of these flavor compounds are called flavan-3-ols, which can be found in high concentrations in cocoa solids (up to 8% by weight). [7,8] Flavan-3-ols have been shown to interact with certain proteins that contain aromatic ring systems, where the flattened networks of delocalized electrons act like Velcro, sticking together and causing interference. [8] CBN is the only tested cannabinoid that contains such an aromatic system; thus, it is proposed that an interaction between CBN and the high levels of flavan-3-ols in the cocoa powder result in a second matrix effect between chocolate and the cannabinoids.
From this study, we can now begin to understand the complexity and nuance of cannabis potency testing. Matrix interference from chocolate, well documented in the food sector, has now been proven with cannabinoid analytes and is shown to be influenced by various factors. Ingredients in chocolate such as fats and cocoa solids increase matrix interference, and higher amounts of these compounds result in more ‘trapped’ analytes and lower recovery rates.
Several structural features of the analyte can also affect the degree of interference: cannabinoids with fewer phenolic –OH groups and/or extended aromatic ring systems both increase the likelihood of substantial matrix interference. Although these newly documented matrix effects have only been confirmed under specific experimental conditions (i.e., three types of chocolate containing five cannabinoids, in methanol), the findings point to the need for widespread study of cannabis potency testing. Chocolate is not the only cannabis-infused matrix that contains these problematic ingredients; fats are found in cookies, brownies, topicals, and butters, and flavan-3-ols can be found in wines, teas, dried fruits, and gummies. Thus, an analogous matrix effect could be at play with any of these compounds. If so, the chocolate matrix interference discussed here could be a harbinger of underlying inadequacies in how we perform potency tests for infused cannabis products. The only way to confirm this hypothesis is with a greater push for peer-reviewed research in cannabis testing, a push that will benefit testing laboratories, cannabis producers, consumers, and the scientific community alike.
References
[1] Thompson A. Which types of Cannabis confectionary edibles are most popular? PMCA event offers insight. Candy Industry website. December 9, 2019. Accessed April 5, 2020. https://www.candyindustry.com/articles/88908-which-types-of-cannabis-confectionery-edibles-are-most-popular-pmca-event-offers-insight[2] Khuda SE, Jackson LS, Fu TJ, Williams KM. Effects of processing on the recovery of food allergens from a model dark chocolate matrix. Food Chem. 2015;168:580-587. [journal impact factor = 6.306; times cited = 25]
[3] Khuda S, Slate A, Pereira M, et al. Effect of processing on recovery and variability associated with immunochemical analytical methods for multiple allergens in a single matrix: dark chocolate. J Agric Food Chem. 2012;60(17):4204-4211. [journal impact factor = 4.192; times cited = 48]
[4] Dawson D, Martin R. Investigation of chocolate matrix interference on cannabinoid analytes. J Agric Food Chem. 2020;68:5699–5706. [journal impact factor = 4.192; times cited = 1]
[5] Drake B. A natural, inexpensive high. In The Marijuana Food Handbook, 2nd ed. Ronin Publishing: Oakland, CA, 2002; pp 17–23.
[6] Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101-106. [journal impact factor = 7.85; times cited = 675]
[7] Afoakwa EO. The chemistry of flavour development during cocoa processing and chocolate manufacture. In Chocolate Science and Technology, 2nd ed.; John Wiley & Sons, Ltd.: West Sussex, U.K., 2016; pp 159–170. [times cited = 204]
[8] Bordenave N, Hamaker BR, Ferruzzi MG. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014;5(1):18-34. [journal impact factor = 4.372; times cited = 208]