A psychoactive non-cannabis non-cannabinoid
Bryophytes are a unique group of land plants arising from an early diverging event some 472 million years ago. [1] They are said to include over 20,000 species, among them mosses, hornworts, and liverworts. Some of their distinctive characteristics include lack of vascular tissue (plant-equivalent of arteries and veins) and favoring moist growing conditions. Notable for our purposes, among the bryophytes, almost all liverworts possess cellular oil bodies, which are membrane-bound cell organelles that consist of ethereal terpenoids and aromatic oils suspended in a carbohydrate- or protein-rich matrix.
Ancient cultures world-wide have traditional medicinal uses for members of the bryophyte class. [2] For example, Native Americans were found to use various mosses in alleviating pain associated with burn injuries, and to form a poultice which promoted osteogenesis in broken bones. Chinese medical texts cite using bryophytes for combatting inflammation, infections, spasticity, and cardiovascular disease. It’s interesting that different species of liverwort have been used and investigated for many of the same medicinal properties we associate with cannabis, with anti-bacterial, anti-microbial, and cytotoxicity against cancerous cells chief amongst them. [1]
Although bryophytes have a long history of medicinal human use, their phytochemistry has been largely neglected over time. [1] One of the reasons as to why is purely logistical; they are of small size, and it’s difficult for botanists to collect sufficient quantities of consistent, high-quality samples in field conditions. Another is that they are difficult to differentiate even under microscopy. Finally, they have always been considered of low nutritional value to human beings, and their biochemistry was therefore not viewed with as much interest as information presented later in this article would suggest it should have been.
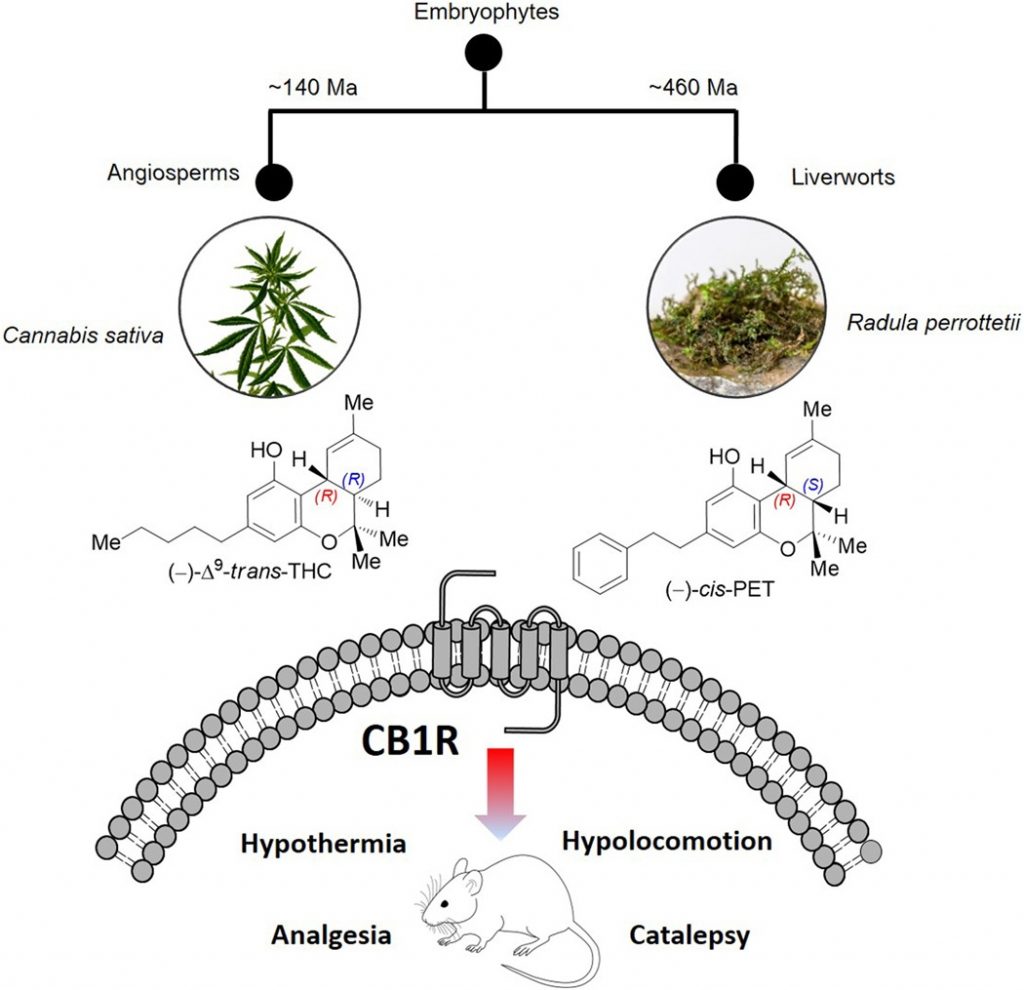
It was generally believed that members of the Cannabis genus were the only plants that produce Δ9–trans-tetrahydrocannabinol, or THC. [3] However, a journal article from1994 reveals that Japanese plant chemist Yoshinori Asakawa discovered a substance in the liverwort plant Radula perrottetii which demonstrates a relation to THC (Figure 1). Dr. Asakawa named this substance perrottetinene in honor of the plant species in which the cannabinoid-like substance was found.
Figure 1. Cannabis plants, belonging to the class of flora known as angiosperms, are separated from liverworts by nearly 460 million years of evolution. It is incredible therefore that these two genera could biosynthesize phytocannabinoids with nearly identical structures using entirely novel and independent catabolic pathways. [3]
The individual atoms in perrottetinene are joined in a similar fashion as those of THC. However, their three-dimensional structures in space differ considerably, as will be discussed below. Furthermore, perrottetinene was found to exhibit an additional benzyl group. [4]
THC in its pure form was first isolated from hashish extract by the “Father of Cannabis,” Dr. Raphael Mechoulam, at the Weizmann Institute of Science,Israel, in 1964. [5]Since then, plants outside the Cannabis genus, closely related members of the Cannabaceae family, as well as more evolutionarily-distant plants, were found to biosynthesize their own exogenous phytocannabinoids (Figure 2). [6]
Figure 2. Phytocannabinoids with similar structures but differing plant origins include CBD (top left) and Ferruginene C(top right). Ferruginene C is produced by the evergreen shrub Rhododendron ferrugineum (bottom right). Image on the lower left shows the CBD-rich cultivar ACDC. [7]
The THC scaffold was presumed to be unique to the Cannabis genus until the discovery ofperrottetinene, which was since clarified to be (−)-cis-perrottetinene (cis-PET). Abibenzyl cis-THC, was isolated from several species of theRadulagenus.[4] Liverworts have been shown to biosynthesize compounds with the opposite stereochemical configuration compared with higher plants like C. sativa. This explains the cis/trans difference seen in the natural three-dimensional stereochemistry of PET compared to THC.
It was demonstrated in vitro that, at extremely low (nanomolar) concentrations, cis-PET selectively binds both CB1 and CB2 receptors without affecting normal central nervous system components or other components of the endocannabinoid system. [4]
In vivo, murine models show thatcis-PET readily crosses the blood-brain barrier and causes the same physiological effects as THC[3], namelyhypothermia, catalepsy, hypolocomotion, and analgesia (collectively called “the tetrad”). [8]
The exciting part in all of this? Both cis- and trans-PET induction led to a surprising and significant decrease in prostaglandin PGD2 and PGE2 levels in the brain. [4] Prostaglandins are a class of molecules that, among normal housekeeping functions, are also responsible for transmitting signals of inflammation and pain. Perrottetinene could therefore be an interesting lead for future drug development, as the reduced prostaglandin levels indicate it may have fewer side effects than associated with THC use.
References
- Asakawa Y1, Ludwiczuk A2. “Chemical Constituents of Bryophytes: Structures and Biological Activity”.J Nat Prod. 2018 Mar;81(3):641-660[Times cited = 11, Journal impact factor = 3.281].
- Ando, H, and Matsuo, A. “Applied bryology”. Advances in Biology. 1984;2:133-224[Times cited = N/A, Journal impact factor = N/A].
- Toyota, M, et al. “Bibenzyl cannabinoid and bisbibenzyl derivative from the liverwort Radula perrottetii”.Phytochemistry.1994;37:859–862 [Times cited = 42, Journal impact factor = 2.547].
- Chicca, A., et al. “Uncovering the psychoactivity of a cannabinoid from liverworts associated with a legal high”. Science Advances. 2018 Oct;4(10) [Times cited = 0*, Journal impact factor = 11.51].
- Gaoni, Y, and Mechoulam, R. “Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish”.J. Am. Chem. Soc. 1964;86(8):1646–1647 [Times cited = 2,214, Journal impact factor = 14.357].
- Gertsch et al. “New Natural Noncannabinoid Ligands for Cannabinoid Type-2 (CB2) Receptors.” J Recept Signal Transduct Res. 2006;26(5-6):709-30 [Times cited = 5, Journal impact factor = 2.2].
- Lee, Ryan. “Appendino’s Advice to Cannabinoid Researchers: Consider ‘New Targets, Chemistry, and Plant Sources’”. International Cannabinoid Research Society: 24th Annual Meeting. O’Shaughnessy’s News Service, June 2014. Retrieved 27 Dec 2018.
- Monory, K, et al.“Genetic dissection of behavioural and autonomic effects of Δ9-tetrahydrocannabinol in mice”. PLOS Biol. 2007;5:e269 [Times cited = 184, Journal impact factor = 9.163].
* Article only published two months ago, natural that citations are limited.