Everyone, from prominent politicians to billionaire business professionals, is lining up to capitalize on the economic value of the cannabis industry. But at Gb Sciences, we believe that cannabinoid-based medicines have significant economic potential, and we have created a therapeutic pipeline of cannabinoid-based therapeutics destined for the prescription drug market, not the cannabis market. Gb Sciences’ novel drug development program leverages the therapeutic potential of cannabinoid-containing mixtures for the treatment of severe medical conditions, and our cannabinoid-drug development programs are among the most advanced in the world.
The principle behind Gb Sciences’ drug discovery research is that plant compounds work better when they are used together rather than as single-ingredient drugs. In the cannabis field, this is known as the “entourage effect,” which comes from a 1998 scientific paper by Professors Shimon Ben-Shabat and Raphael Mechoulam titled “An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity.” [1] The original use of the term “entourage effect” cast just tetrahydrocannabinol (THC) as the star with other cannabinoids and fatty acids as supporting cast members. However, a 2011 review by Dr. Ethan Russo, “Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects,” changed the original entourage paradigm to include both cannabinoids and terpenes with any of these potentially in the starring role, which describes more of an ensemble cast. [2]
Today, the “entourage effect” is the general theory that both cannabinoid and terpene molecules from cannabis extracts can act synergistically on target receptors in human patients. We use the term “molecular synergies” to describe this same powerful potential for increased efficacies within optimized therapeutic mixtures (OTM) relative to the effectiveness of the same ingredients being used individually.
We have developed a novel drug discovery program to identify mixtures of cannabis-derived ingredients that demonstrate molecular synergies in the treatment of specific human disorders including Parkinson’s disease, chronic pain, COVID-19-related cytokine release syndrome, and heart disease, among many others. We combine natural product research with our proprietary artificial intelligence-enabled drug discovery engine, PhAROSTM (Phytomedicine Analytics for Research Optimization at Scale), to rapidly identify potentially effective combinations of plant-derived ingredients for specific diseases. We test these plant-based mixtures using rigorous, high-throughput cell and animal models of the specific disease.
Our product formulas are OTM designed to reduce the motor symptoms of Parkinson’s disease. We started with a database containing hundreds of chemical profiles from different cannabis varieties and used in silico screening techniques to reduce the number of starting combinations of active ingredients from these cannabis metabolomes from well over 100,000 mixtures to just 1,080 mixtures that were screened in two different cell models of Parkinson’s disease. The cell-based experiments demonstrated molecular synergies between the active ingredients in our OTM relative to the individual components tested separately.
The demonstration of molecular synergies in these OTM formed the basis for the US patent that was issued on using these mixtures for the treatment of Parkinson’s disease in 2020. The cell screening experiments further reduced the numbers of potential therapeutic combinations to 24 mixtures, based on their efficacy in the cell experiments. The 24 mixtures were then tested in a zebrafish model of Parkinson’s disease. Of the 24 OTM tested, 3 OTM achieved a statistically significant reduction in Parkinsonian motor symptoms. We then performed additional experiments to optimize the ratios of active ingredients in these top three OTM for Parkinson’s disease that are being formulated for a first-in-human clinical trial.
We’ve currently turned our attention to the lingering COVID-19 pandemic, and we have tested cannabis-based ingredients alone as well as in OTM to assess their ability to reduce hyperinflammation and cytokine release syndrome (CRS). Our COVID-related CRS mixtures are designed to reduce the life-threatening levels of specific cytokines and pro-inflammatory processes triggered by COVID-19 (the effects of the SARS-CoV-2 virus), while preserving the immune functions and cytokines necessary for fighting SARS-CoV-2 and other opportunistic viral infections found in hospitals. We were trying to design a “selective” anti-inflammatory therapy utilizing the synergies among cannabis-based ingredients, and the results from our proof-of-concept study were very positive.
The proof-of-concept study was performed at Michigan State University using a state-of-the-science human immune model. Immune cells from human donors were co-cultured together in one of four treatment groups: untreated (no inflammatory stimulus), inflammatory stimulus, control (inflammatory stimulus + vehicle from cannabinoid mixtures), or pre-treatment with the cannabinoid mixture + inflammatory stimulus. Then, a panel of cytokines and inflammatory markers was measured from each of these treatment groups from different immune cell types within the co-cultured cells at four time points to determine whether the cannabinoid mixtures were able to alter the levels of pro-inflammatory cytokines or other inflammatory agents.
All twenty-four of Gb Sciences’ OTM achieved statistical significance in modulating cytokine levels in at least one of the assays within the panel of cytokines and inflammatory markers in at least one of the immune cell types measured. The twenty-four OTM were divided into functional categories based on the selectivity of their anti-inflammatory activities. Eight of the OTM demonstrated selective anti-inflammatory activity, and sixteen of the OTM demonstrated broadly immunosuppressive activity.
The eight selective anti-inflammatory OTM met our ultimate performance criteria: they suppressed COVID-related CRS but left key antiviral immune functions intact to help in the fight against opportunistic infections. The best performing OTM will be further developed in preparation for clinical studies to evaluate their anti-inflammatory potential in the treatment of severely ill COVID-19 patients contending with CRS and associated hyperinflammatory conditions, such as macrophage activation syndrome (MAS) and acute respiratory distress syndrome (ARDS). CRS, MAS, and ARDS are the leading causes of deaths in COVID-19 patients.
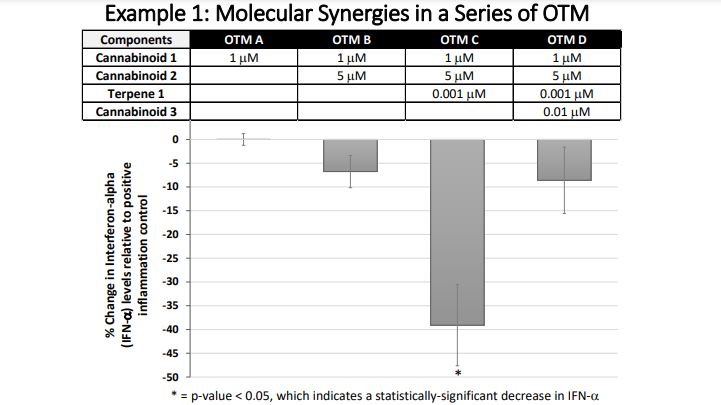
The results from this proof-of-concept study also elegantly demonstrate the principles behind molecular synergies (ensemble effects). In these experiments, we tested the anti-inflammatory effects of single ingredients as well as mixtures of 2, 3, 4, or 5 ingredients. By looking at the changes in the effectiveness of the mixtures as ingredients are added in succession, it becomes clear that the number of and kinds of ingredients in these mixtures dramatically affect the anti-inflammatory activity of the mixture. As demonstrated in example 1, a double ingredient mixture like OTM B may be trending towards statistically significant anti-inflammatory activity, but then a small amount of another ingredient will dramatically increase the anti-inflammatory activity that is measured in the combined mixture as seen for OTM C. In example 1, OTM C caused a statistically significant decrease in the pro-inflammatory substance, interferon-alpha. However, the addition of a fourth ingredient in OTM D changed the anti-inflammatory potential that was measured in OTM C. OTM D did not show a statistically significant reduction in IFN-alpha, like in OTM C. Therefore, more ingredients are not always more effective in an OTM.
In example 2, we compare the effects of two mixtures (OTM E and OTM G) that are composed of the same three ingredients, but with those ingredients at different relative concentrations. We also add the same amount of another cannabinoid to these three component mixtures (OTM E and OTM G) which produces opposite effects in the resultant mixtures, OTM F and OTM G, respectively. Even though OTM E and OTM G have the same compounds in them, only OTM G with a lower concentration of Cannabinoid 2 causes a statistically significant reduction in IFN-alpha. These results highlight the importance of not only which ingredients are in a therapeutic mixture, but also the relative abundance of the ingredients in the mixtures for establishing an effective OTM. In Example 2, OTM F is created by adding 0.01 micromolar of Cannabinoid 5 to the non-reactive OTM E. The resultant OTM F caused a statistically significant reduction in IFN-alpha levels. In contrast, OTM H is created by adding 0.01 micromolar of Cannabinoid 5 to the significantly anti-inflammatory OTM G. However, the resultant OTM H did not cause a statistically significant reduction in IFN-alpha levels. The addition of 0.01 micromolar of Cannabinoid 5 obliterated the high anti-inflammatory potential of OTM G. These results highlight the fact that molecular synergies can be either positively or negatively reinforcing.
We believe in the potential of optimized therapeutic mixtures for the treatment of human disorders; however, these mixtures of plant-based compounds require extra effort on the formulation and testing side of drug development. Combining precise delivery formats and diligent research efforts to our OTM, we aim to bring defined mixtures of cannabinoids to pharmaceutical markets beyond state-regulated cannabis markets.
About Gb Sciences
Gb Sciences, Inc. (OTCQB: GBLX) is a plant-inspired biopharmaceutical development company creating patented, disease-targeted formulations of optimized therapeutic mixtures of plant-based compounds for the prescription drug market through GbS Global Biopharma, our Canadian subsidiary. The “plant-inspired” active ingredients in our therapeutic mixtures are synthetic homologues identical to the original plant compounds but produced under current Good Manufacturing Practices. Gb Sciences’ novel drug discovery platform has yielded five issued U.S. and three issued international patents, as well as 19 U.S. and 40 international patent-pending applications. In our drug development pipeline, we have four preclinical-stage programs, and our lead Parkinson’s disease therapeutic program is being prepared for a first-in-human clinical trial. In addition to Parkinson’s disease, we are developing therapeutics for neuropathic pain, COVID-related CRS, mast cell activation syndrome (MCAS), and heart failure. Gb Sciences’ productive research and development network includes distinguished universities, hospitals, and contract research organizations. https://gbsciences.com
References
[1] Ben-Shabat S, Fride E, Sheskin T, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353(1):23-31. [journal impact factor = 4.432; times cited = 451] [2] Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344-1364. [journal impact factor = 8.739; times cited = 763]