Mold and Mycotoxins
Moldy cannabis can kill you [1], give you cancer [2], or lead to other permanent and severe illnesses like liver damage [3]. The poisonous chemical components of molds found in crops like cannabis, wheat, rice, and corn are called mycotoxins. A few hundred mycotoxins exist, and they are difficult to detect in their naturally complex fungal matrix (Figure 1).
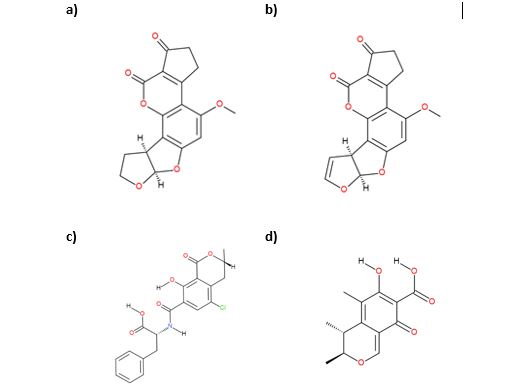
Figure 1 – Aflatoxin type mycotoxins found in Cannabis. Chemically similar Aflatoxins: a) Aflatoxin B1, and b) Aflatoxin B2, and other mycotoxins c) Ochratoxin A, and d) Citrinin.
They are incredibly poisonous in small quantities and render foodstuff like butter, cheese, and cocoa inedible. [4] Improper testing, storage, or growing conditions can lead to the formation of mold in cannabis because of the naturally warm and humid environment required for healthy cannabis growth. [5] One might suspect poisonous mycotoxins are destroyed while burning cannabis for inhalation; on the contrary, there is evidence that inhaling mycotoxin-contaminated material can result in pulmonary emphysema and lung cancer. [6] Moldy cannabis products are unacceptable for health reasons and more work is required for best practices in testing. Here, we outline some of the analytical challenges of mycotoxin detection in cannabis and what is required moving forward.
Figure 2 – Fungal mold growing in a petri dish. (Esv, CC BY-SA 3.0 via Wikimedia Commons)
Need for Vigilant Regulation
In November 2018, one month after legalization in Canada, a licensed producer was forced to recall several products from cannabis stores due to customer reports of mold and bugs. [7] Despite best efforts to avoid mycotoxins, the incident cast doubt on the ability to regulate the product and protect customers. [8] Unfortunately, moldy products continue to cause economic waste because of flower and edible product recalls.
In the United States, individual states including Maryland [9], Nevada [10], and Michigan [11] have implemented specific testing methods whereas elsewhere in the country, i.e., California and Alaska, the protocols are less stringent and do not mandate mycotoxin testing [12]. As testing regulations develop from region to region, improved mycotoxin assays for early detection and accurate quantification are required.
Analytical Challenges
A solid analytical methodology should start with reliable quantification of regulated mycotoxins and expand the library of targets over time. There are a few hundred known mycotoxins of concern in cannabis testing. Accurate quantification of all toxic compounds present in cannabis should be measured; however, this traditionally requires an expensive pure standard for each analyte. Mycotoxins are exotic chemicals and are difficult to produce and purify. For this reason, targeted analyses must prioritize which mycotoxins to quantify.
Due to the complex nature of plants, it can be tedious to characterize mycotoxins. A recent study demonstrated the impact of sample matrix which hinders accurate mycotoxin quantification. [13] The matrix refers to where the analyte to be quantified resides – in cannabis, the matrix is plant matter. In edibles, the matrix could be chocolate, gummies, or oil. In the study, a considerable amount of matrix interference masked mycotoxin concentration. To overcome this, the researchers employed mass spectrometric isotope dilution experiments which measure relative amounts of extremely similar species and effectively overcome sample matrix interference. Using Carbon-13 chemical analogues of the mycotoxins renders this method high-priced and thus, it can only be performed by well-funded labs. An acceptable alternative is “matrix matching” in which calibrants are prepared to match the chemical matrix of the samples. This is laborious, requires expensive mycotoxin calibrants, and is not ideal in all cases or for all mycotoxins. Methods used to avoid matrix effects (and other analytical errors) are a balancing act between cost, time, accuracy, and precision, and will vary from lab to lab.
An additional challenge in obtaining accurate mycotoxin results is analyte masking. Masking is a process in which mycotoxins are functionalized with chemical moieties causing them to appear as something else. In cannabis, for example, this could present itself as glycosylation. [14] Preparative steps to cleave the added functionality from the target molecule could compensate for analyte masking but must be considered carefully so as not to introduce new interferences.
Figure 3 – Example of a glycosylated mycotoxin, deoxynivalenol-3-O-β-D-glucoside
Techniques in Mycotoxin Analysis
Mycotoxin analysis is tricky, and we face new challenges with cannabis products that are slightly different from existing solutions found in food testing. However, we have a combination of advanced methods to attack the problem. Mycotoxin quantification undoubtedly requires a combination of analytical techniques. Liquid and gas chromatography systems are industry workhorses for achieving chemical separation and a requirement in any serious testing laboratory. Separation is typically followed by sensitive detectors. Spectroscopic and spectrometric detectors like UV-Vis spectrophotometers and quadrupole-time-of-flight (qTOF) high-resolution mass analyzers pair well together by yielding complementary information. [15,16] Cost of equipment is certainly a consideration and additional research effort should be focused on improving inexpensive, accurate, and reliable tests. Because of prohibitively expensive chemical standards, advanced statistical techniques for the analysis of untargeted data along with orthogonal detection strategies will greatly improve our understanding of mycotoxin formation in cannabis and will help reduce cost of analysis.
Take Home Message – What About Consumers?
The risk of mycotoxicosis is significant even with government oversight. Cannabis regulators and researchers must be conscious of modern analytical methods to decide on an acceptable level of mold and mycotoxins. There is general agreement that less than one part per billion is acceptable to minimize adverse health effects. [17] We can learn lessons from food industries in which mycotoxins are historically a problem to study, and implement mycotoxin and mold suppression techniques which have proven effective.
Figure 4a: A moldy bud of cannabis. (Source: Anargratos, CC BY-SA 4.0 via Wikimedia Commons); Figure 4b: Cannabis leaf with signs of powdery mildew (bud rot / gray mold). Source: Whitney Cranshaw, Colorado State University, Bugwood.org.
In the meantime, there are a few things we can work on as consumers. Visual inspection for mold on products is a good start since this could indicate the presence of mycotoxins. Discard purchased products containing mold but first make note of the LOT# and report the incident. Know what you are looking for (Figure 4), know how to store the material, and inform your favorite licensed producer that you expect quality assurance testing protocols for mycotoxin and mold are in place.
References
[1] Thompson GR 3rd, Tuscano JM, Dennis M, et al. A microbiome assessment of medical marijuana. Clin Microbiol Infect. 2017;23(4):269-270. [journal impact factor = 7.117; times cited = 40][2] Ostry V, Malir F, Toman J, Grosse Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017;33(1):65-73. [journal impact factor = 1.354; times cited = 215]
[3] World Health Organization. Mycotoxins. Posted May 9, 2018. Accessed May 11, 2021.
[4] Taniwaki MH, & Pitt JI. Mycotoxins. Food Microbiology: Fundamentals and Frontiers. Eds: Doyle M, Diez-Gonzalez F, Hill C. ASM Press: Washington DC. 2019. 22:585-608.
[5] Wilcox J, Pazdanska M, Milligan C, Chan D, Macdonald S, & Donnelly C. Analysis of Aflatoxins and Ochratoxin A in Cannabis and cannabis products by LC-fluorescence detection using cleanup with either multiantibody immunoaffinity columns or an automated system with in-line reusable immunoaffinity cartridges. Journal of AOAC International. 2020;103(2):494–503. [journal impact factor = 1.36; times cited = 4]
[6] Forgacs J, Carll WT. Mycotoxicosis: toxic fungi in tobaccos. Science. 1966;152(3729):1634-1635. [journal impact factor = 41.845; times cited = 30]
[7] Kalvapalle R. (2018, November 24). RedeCan recalls pot from Ontario Cannabis Store amid reports of mould, bugs. Global News website. Posted November 24, 2018. Accessed May 11, 2021.
[8] Government of Canada. Guidance document: good production practices guide for cannabis. Published August 29, 2019. Accessed May 11, 2021.
[9] Dodson L, Laprade NM. The Natalie M. Laprade Maryland Medical Cannabis Commission’s (MMCC) Technical Authority for Medical Cannabis Testing. Maryland Medical Cannabis Commission website. Published November 15, 2019. Accessed May 11, 2021.
[10] Chapter 453D – Adult use of marijuana: Requirements for marijuana testing facilities. Accessed May 11, 2021.
[11] Marijuana Regulatory Agency. Rule 31. Testing; safety compliance facility. Michigan.gov website. Accessed May 11, 2021.
[12] Cannabis testing regulations: A state-by-state guide. Leafly website. Updated February 24, 2020. Accessed May 11, 2021.
[13] Zhang K, Schaab MR, Southwood G, et al. A collaborative study: Determination of mycotoxins in corn, peanut butter, and wheat flour using stable isotope dilution assay (SIDA) and liquid chromatography-tandem mass spectrometry (LC-MS/MS). J Agric Food Chem. 2017;65(33):7138-7152. [journal impact factor = 4.192; times cited = 26]
[14] Michlmayr H, Malachová A, Varga E, et al. Biochemical characterization of a recombinant UDP-glucosyltransferase from rice and enzymatic production of deoxynivalenol-3-O-β-D-glucoside. Toxins (Basel). 2015;7(7):2685-2700. [journal impact factor = 3.531; times cited = 28]
[15] Fiehn Lab. Mass resolution and resolving power. UC Davis – Fiehn Lab website. Accessed May 11, 2021.
[16] DiNardo FD, Cavalera S, Baggiani C, Chiarello M, Pazzi M, Anfossi L. Enzyme immunoassay for measuring Aflatoxin B1 in legal cannabis. Toxins (Basel). 2020;12(4):265. [journal impact factor = 3.531; times cited = 2]
[17] Pichler E. Sampling for mycotoxins – do we care enough? Romer Labs website. Posted July 12, 2016. Accessed May 11, 2021.
Bios:
Dr. Eric Janusson is an analytical organometallic chemist specializing in real-time methods for quantification and characterization of sensitive chemical intermediates. His research interests include machine learning, statistical modelling, organometallic catalysis, nanoparticle chemistry, automated synthesis, ion mobility, and mass spectrometry. He enjoys robotics and programming and other advanced tools used to solve complex problems. Dr. Janusson received his Ph.D. in Analytical Chemistry from the University of Victoria in 2017, held a postdoctoral scholar position at the University of Glasgow in Scotland until 2019, and is now spearheading the chemical analysis efforts of Complex Biotech Discovery Ventures in Vancouver, Canada.
Ali Wasti is a Chemical Engineering student at the University of British Columbia minoring in commerce and specializing in the process stream of analytical chemistry combined with mechatronics. His studies include thermodynamics, reactor design, reaction kinetics and process development integrating that with organizational behavior and marketing management he believes that this combination of fields can lead to further progress in his career. Ali is keen to explore new realms of modern technology and method development by using resources on hand. Recently, his research in analytical chemistry involved spectroscopic-led investigation of geological samples, where he regulated quality assurance and quality control, as well as the development of mass spectrometric methods at his current coop position at Complex Biotech Discovery Ventures where he mainly works with cannabis and psychedelic compounds to discover new possibilities and provide innovation. Ali aims to complete his undergraduate in the following year and enhance his engineering skills to prosper the industry.
Dr. Markus Roggen’s latest project, Complex Biotech Discovery Ventures, is a fundamental research laboratory and CRO for the cannabis, hemp and psychedelic mushroom industries. His research interests lie in the metabolite composition and behavior throughout the production cycle, extraction optimization, and development of innovative therapeutic formulations. Dr. Roggen received his M/Sci degree from Imperial College, London, UK in 2008. He then pursued his graduate degree in organic chemistry at the Federal Institute of Technology in Zürich (ETHZ), where he received his PhD in 2012. Dr. Roggen was awarded an DAAD postdoctoral fellowship to pursue further training in physical organic chemistry at The Scripps Research Institute in La Jolla from 2013-2014. He then entered the cannabis industry, at first as laboratory director for Davinci Laboratories of California, an analytical laboratory from 2014 to 2016. In 2016, he moved into an executive position overseeing production, R&D and process optimization for OutCo, a vertically integrated cannabis company. Dr. Roggen is also a trusted advisor and mentor to multiple startups, startup accelerators and organizations. Positions include advisory positions at Bloom Automation, a cannabis robotics company, and former SAB member at MediPharm Labs, a Canadian LP, and co-chair of the NCIA Scientific Advisory Committee.