If you survey analytical chemists in the cannabis industry regarding which instrument they wish they had access to, nuclear magnetic resonance (NMR) spectroscopy will no doubt be high on the list. This is due to NMR’s ability to identify unknown constituents, something many product manufacturers would like to achieve. As we learn about cannabis and the compounds therein, the need to identify closely structured molecules is increasingly important and NMR can provide a powerful tool.
But reading about NMR can be esoteric and daunting. The discussions of magnetic moments and spins can leave one dizzied. This blog seeks to simplify this complex technique.
We flirted with NMR during an interview with organic chemist and industry consultant, Josh Jones, Ph.D., who utilized NMR to elucidate the structure of an unknown precipitate captured during distillation. While chromatography pointed to cannabichromene or even cannabicyclol, NMR demonstrated that the compound was likely delta-10-tetrahydrocannabinol, which was later confirmed using chromatographic methods.
So, NMR can help identify the chemical structure of an unknown compound. But how does this all work?
NMR Basics
Electrons, protons, and neutrons are subatomic particles that comprise an atom. The nuclei of atoms with an odd mass or atomic number (1H, 13C, 31P) have nuclear spin. This occurs when there’s not the same number of protons as neutrons, such as carbon-13 with its 7 neutrons and 6 protons. How utterly isotopic.
You can envision these particles spinning on their own axes just as does our Earth at about 1,000 miles per hour. These odd atomic nuclei are said to possess a “magnetic moment” which is analogous to a molecular dipole moment where there are localized regions of positive and negative charges (like in H2O). The magnetic moment just means that the spins are not balanced between spin-up and spin-down levels as they are in an atom like carbon-12, just like the dipole moment where the positive and negative charges are not net neutral.
Still with me?
When atoms with these types of nuclei are placed within a strong magnetic field, they precess like a toy top (Figure 1). Precession is what enables NMR spectroscopy. Now it gets weird.
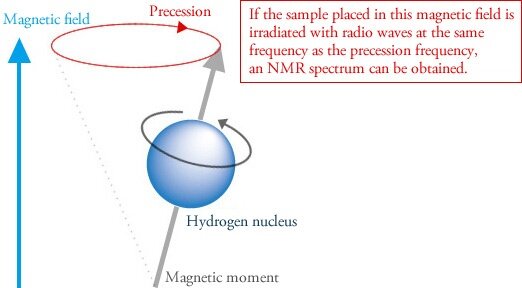
Figure 1
A sample is just a collection of atoms, right? Some of these atoms will be NMR active as defined above. Their tiny magnetic moments organize and coalesce into one overall alignment. If you place your sample — which has its characteristic precession frequency — into a magnetic field, and irradiate with radio waves at a resonating frequency, you’ll get an NMR spectrum, such as for a hemp extract in ethanol (Figure 2). The radio frequency applied is depicted on the x-axis, while the absorption of this energy is shown on the y-axis.

Figure 2: Typical 1H spectrum of a hemp ethanolic extract. Image used with permission from Marchetti L, Brighenti V, Rossi MC, Sperlea J, Pellati F, Bertelli D. Use of 13C-qNMR Spectroscopy for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis sativa L. (Hemp). Molecules. 2019;24(6):1138. [1]
Like other types of spectroscopy, irradiating a sample with energy can bring about an energy transfer. In NMR spectroscopy, the radio waves engage the sample, energy is transferred to the sample, and when the spin returns to its ground state, energy is released. The resonant frequency of this energy transition is directly related to a nucleus’ chemical surroundings. Electron clouds can shield the nucleus from the incoming energy, and electronegative nuclei tend to have higher resonant frequencies.
The frequency of the measured signal is normalized to a reference standard (e.g., trimethylsilane) defined as 0 parts per million. This is known as the chemical shift. The resonant frequency is dependent on the magnetic field used and can be a long series of numbers (e.g. six decimal places), so to simplify the data, the chemical shift was adopted.
Because cannabinoids have very similar structures, some instrumental methods will be hard-pressed to differentiate them. NMR, however, can help distinguish subtle chemical differences, such as C5 side-chain alterations, cyclization, or substitutions of a carboxylic acid and hydroxyl (OH) group. [2] Using multiple NMR techniques, researchers were able to identify Δ9-tetrahydrocannabinol, tetrahydrocannabinolic acid, Δ8-tetrahydrocannabinol, cannabigerol, cannabinol, cannabidiol, cannabidiolic acid, cannflavin B, and cannflavin A, all extracted from cannabis flowers. [2]
Image credits: David Cardinez from Pixabay; JEOL USA
References
- Marchetti L, Brighenti V, Rossi MC, Sperlea J, Pellati F, Bertelli D. Use of 13C-qNMR spectroscopy for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (Hemp). Molecules. 2019;24(6):1138. [journal impact factor = 3.267; times cited = 2 (Semantic Scholar)]
- Choi YH, Hazekamp A, Peltenburg-Looman AM, et al. NMR assignments of the major cannabinoids and cannabiflavonoids isolated from flowers of Cannabis sativa. Phytochem Anal. 2004;15(6):345-354. [journal impact factor = 2.772; times cited = 71 (Semantic Scholar)]








