Intro to Minor Cannabinoids
‘Minor’ cannabinoids, or just ‘minors’, are becoming all the rage, especially in the hemp and smoke shop markets. These cannabinoids are uniquely identified in Cannabis sativa but expressed in amounts too small for commercial extraction and purification. Cannabis is a remarkable factory of diverse bioactive small molecules, producing over 120 different phytocannabinoids, with minor cannabinoids representing much of that diversity. [1] As the bioactivities of minor cannabinoids are significantly different from cannabidiol (CBD) and delta-9-tetrahydrocannabinol (delta-9-THC), minors have gained recognition as curiosities and as putative contributors to the entourage effect. [2] As producers seek new value in a now familiar crop, these rare plant metabolites are entering the spotlight with consumers eager to explore more of what Cannabis has to offer.
‘Major’ cannabinoids, including cannabigerolic acid (CBGA), tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), and cannabichromenic acid (CBCA), already represent billions of dollars of annual economic impact. Yet these four, well-known cannabinoids are still just the tip of the iceberg for what’s in store in this era of widespread cannabinoid consumption upon us. And this is the appeal of the minors: just look at the impact from commercializing only four major cannabinoids, then imagine what the next 20 could bring. But without suitable cultivars, accessing bulk quantities of minors from plant extracts isn’t yet feasible. So, with an increasing supply of minor cannabinoids entering the market, how are they being produced and what does the future hold?
Market Demand: Consumer Interest
Consumers of hemp products, perhaps familiar with CBD, CBG and CBC and interested in exploring the entourage effect much touted in the cannabis industry (albeit with terpene diversity in THC-rich cannabis versus hemp’s cannabinoid diversity as source of entourage) [3], have yearned to explore new effects of minor cannabinoids, especially those providing more of a perceptible ‘feeling’ than CBD, be it well-being, relaxation, increased mental or physical focus, pain relief, or even mild intoxication.
However, full-spectrum hemp products containing federally compliant levels of THC (<0.3%), have already provided hemp consumers with intoxicating potential (e.g., 5 mL of a tincture containing 0.3% THC equates to 15 mg of THC.) So, intoxication alone is not what’s driving demand for minor cannabinoids. More than anything, demand represents an appeal to plant-based medicines, something the cannabis industries as a whole have renewed. [4] Concurrent with the rise of minor cannabinoids, the Food and Drug Administration’s approval of Epidiolex with CBD from hemp as the active ingredient seemed a validation of plant-based medicines to consumers searching for non-pharmaceutical treatments of disruptive conditions not otherwise effectively managed. While CBD isn’t now considered a minor cannabinoid, it was exactly that before breeders succeeded in minimizing THC and maximizing CBD in available cultivars. More recently, breeders have achieved higher concentrations of cannabidivarin (CBDV), increasing its availability to consumers. But the breeding process isn’t quick, so producers are exploring a range of methods to bring more minors to market (vide infra). For the many other minors expected to come, a growing wealth of animal and human studies is available to guide the interests of educated consumers in the ‘cannabinoid of the month.’
Market Demand: Producer Interest
Meanwhile, during FDA’s review and approval of Epidiolex, hemp processors were flooded with hemp to extract and in turn flooded the market with CBD, increasing competition for what was widely expected to soon be co-opted by the largest of multi-national consumer product brands. However, Congress and the FDA’s ongoing resistance to approve CBD, or any phytocannabinoid, as a dietary supplement, caused billion-dollar brands to pull-out of late-stage deals to formulate and distribute CBD products. This exacerbated an already competitive wholesale CBD market, and producers faced a race-to-the-bottom for what so recently had been highly profitable (CBD’s wholesale value decreased >97% over 5 years). The crash of CBD prices, combined with a growing consumer demand for new products derived from hemp, created opportunity for the production of minor cannabinoids. But with few minors economically grown, extracted, and standardized into consistent products, hemp processors began exploring the chemical conversion of CBD into CBN and various, unregulated isomers of THC. Such conversions, primarily achieved semi-synthetically, first provided wide access to several high-value, nature-identical cannabinoids not otherwise available in standardized potencies from hemp extracts. With at least 120 known phytocannabinoids but less than 10 identifiable in commonly available Cannabis extracts, the pursuit of minor cannabinoids and exploration of their therapeutic and wellness potential is only just beginning.
Availability & Effects of Minors
As readers will likely know, despite decades of structured research into the bioactivity of phytocannabinoids, funding and permission in the USA to perform such research has largely been limited to demonstrating the drug abuse potential of Δ9-THC. Nevertheless, and because of renewed interest in the non-abusive and therapeutic use of cannabinoids [5], a more nuanced picture of their diverse effects in humans is developing. Minor cannabinoids, in part due to their largely unscheduled status with the DEA, are now a focus of researchers and the consumer market aiming to discover value in cannabinoids beyond the well-established intoxicating potential Δ9-THC. [6] Some minors have been available for several years, especially those either available from the plant or with practical synthetic or semi-synthetic methods already known. [7] Those ‘early bloomers’, like CBDV [8-10], cannabinol (CBN) [11], and Δ8-THC [12], were followed more recently by cannabigerolic acid (CBGA) [13] and hexahydrocannabinol (HHC). [14] These have each been turning points in the hemp market, catching consumers’ interests with the variety of effects available.
Δ8-THC, or just ‘Δ8’, deserves a special mention, due both to its prevalence on the market and its mildly intoxicating effects. Consumers quickly found a middle ground in Δ8, something between strongly intoxicating Δ9-THC and the non-intoxicating effects of CBD. Because Δ8 is largely derived (albeit chemically) from hemp CBD, it is formulated into various hemp products and made available nationwide. This injected the hemp industry with fresh capital at a time when all seemed lost, and further whetted the appetites of producers and consumers for what would come next. Furthermore, similar to the production of CBN, Δ8 familiarized hemp producers with the techniques of synthetic chemistry. As extraction facilities morphed into natural products laboratories, a manufacturing infrastructure was being built for versatile access to virtually any cannabinoid.
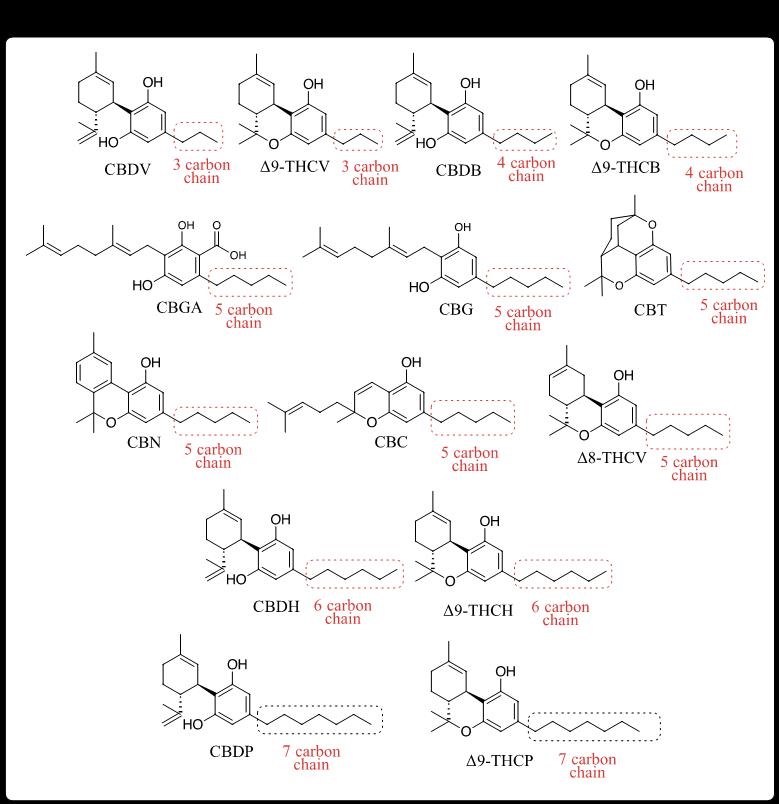
With Δ8 leading the charge, and with a steady inflow of academic discoveries unveiling previously unknown minors, producers have been fast on the trail, bringing them to market almost as quickly as they have been discovered (Figure 1). A structural trend in these recently available minors is the variation in side-chain length of CBD and THC, with each change in length referred to as a homolog. For example, tetrahydrocannabivarin (THCV) [10,15] is a 3-carbon homolog of the 5-carbon THC. The effect of side-chain length on intoxicating potential is profound, with shorter chains having less of an effect and longer chains having more. Accordingly, Δ9-THCV lacks significant intoxication compared to Δ9-THC. Longer chain homologs, like tetrahydrocannabiphorol (THCP) with 7 carbons, binds more tightly to human endocannabinoid receptor 1 (CB1R), resulting in increased potency and duration of intoxication. [16] Other THC homologs, with 4 carbons (tetrahydrocannabutol, THCB [17]) or 6 carbons (tetrahydrocannabihexol, THCH [18]) fall somewhere in the middle. But a lack of formal studies on many of these homologs leaves effects to be reported informally. On the non-psychoactive front, the bioactivity of CBD homologs, like cannabidibutol (CBDB) [19], cannabidihexol (CBDH) [20], and cannabidiphorol (CBDP) [191, 16] are largely unknown and are scarcely seen on the market. Perhaps because human endocannabinoid 2 receptors (CB2R) are more widely distributed in the body and with more diverse effects compared to CB1R’s relative isolation in the nervous system, it will take more time to better understand their unrecognized activities. However, it’s interesting to note that CBDV, with its short 3-carbon chain, has significant binding affinity to various receptors [8], so the trend of intoxication seen in THC homologs may not be similarly reflected in the CBD homologs.
Production of Minor Cannabinoids
Botanical Production
The increased presence of minor cannabinoids in some Cannabis chemotypes is a valuable trait and opportunity for breeders to coax these minor traits into major metabolites. Over the past 50 years, selective breeding of Cannabis has boosted common levels of both (-)-trans-Δ9-tetrahydrocannabinol (THC) and (-)-cannabidiol (CBD) to levels not previously seen, allowing the cannabis industries to flourish. These successful breeding efforts, perhaps as a template for boosting levels of other cannabinoids, have demonstrated the power of selective breeding to dramatically improve production efficiencies for cannabinoids previously considered ‘minor.’ [2]
Synthetic Biology
With commercial cannabis varieties bred to express only a few major cannabinoids, the associated genetic sequences have been understood to the extent that their production has been achieved using synthetic biology. [21] This approach, commonly applied to commercialize difficult-to-access natural products (e.g., insulin) [22] , uses genetic modification of yeasts (most frequently) to insert genes for producing the desired product, thereby allowing the host’s metabolic processes to do the work. This approach has provided numerous cannabinoids [23], including all the major cannabinoids and some analogs like CBDV. While still a work in progress at commercial scale [24], even a small amount of cannabinoids produced in heterologous hosts (e.g., yeasts) indicates good understanding of the enzymes cannabis uses to produce these cannabinoids, which is a feat major in itself. More experience is being gained for a versatile application of synthetic biology to produce cannabinoids. Until such time when synthetic biology can compete with either botanical or synthetic production of minor cannabinoids, organic chemistry remains an established method for bringing these compounds to market.
Semi-synthesis
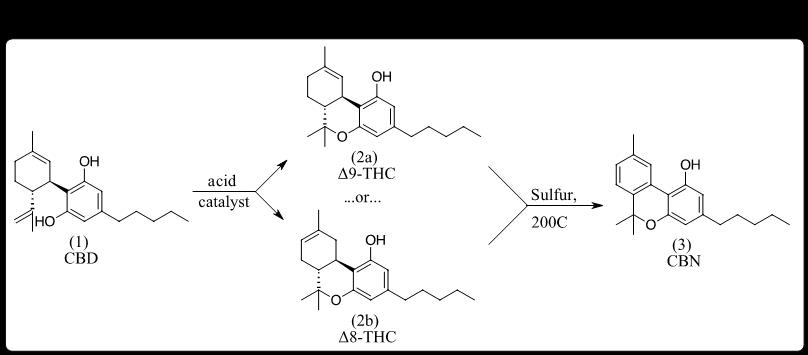
Enter the Chemist. Chemistry, especially organic chemistry, has played a pivotal role in evolving the science and industries of Cannabis. This is due, in part, to the collected works of Raphael Mechoulam, a prolific Professor of Chemistry who established the molecular structure of THC [25] and described the conversion of CBD into various positional isomers of THC (e.g., Δ8-THC, Δ9-THC, Δ10-THC). Many other chemists, both before and after Mechoulam, had laid the groundwork for hemp producers to consider various semi-synthetic conversions of CBD into minor cannabinoids not readily accessible from plant extracts. [26] By 2018, CBD isolate was widely available in purities >95%, providing starting material for conversion into more valuable product ingredients. Soon, cannabinol (CBN), long known as accessible by the treatment of THC with sulfur (Figure 2) [12], was likely the first minor cannabinoid semi-synthetically derived from CBD to enter the hemp market in bulk, crystalline, purified form.
This CBN was initially produced using THC, either converted from CBD or separated from ‘hot hemp’ extracts to make them compliant with the federal 0.3% Δ9-THC limit in hemp products. Initially selling in bulk for $50,000/kg, CBN caught the eye of many hemp processors seeking to maintain revenues as CBD prices began their fall. Shortly thereafter, an academic article described the direct conversion of CBD to CBN using iodine, which, when applied at commercial scale, enabled access to CBN without having to go through THC as an intermediate. [27]
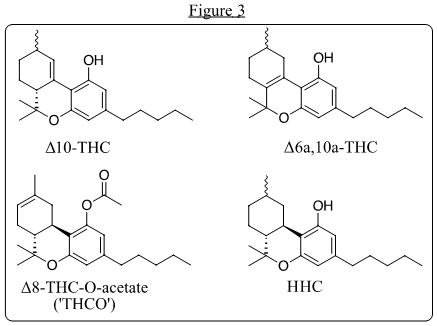
With THC no longer a necessary intermediate to CBN, and with producers already converting CBD to Δ8-THC for CBN, surplus Δ8-THC entered the market, with consumers welcoming a mildly intoxicating alternative to more potent (and more heavily regulated) Δ9-THC from THC-dominant cannabis. The market for hemp-derived Δ8-THC quickly ballooned to a peak production of more than 10 metric tons per month (USA). This provided steady revenue for hemp farmers eager to unload crop unsellable if used only for CBD. A price war swiftly ensued amongst Δ8 producers and within a year the ‘Δ8 craze’ was followed by conversions of CBD to Δ10-THC and Δ6a,10-THC also using methods based on Mechoulam’s work. These were accompanied by hexahydrocannabinol (HHC) and Δ8-THC-O-acetate, a functional analog and prodrug of Δ8-THC (Figure 3).
Total Synthesis
Despite ongoing efforts to produce a diversity of cannabinoids through enzyme-based systems, whether in the plant or through synthetic biology, many minor cannabinoids are more feasibly produced by ‘total synthesis’, meaning through the methods of synthetic organic chemistry. Conveniently, a wealth of methodology already exists for the total synthesis of several cannabinoids, although most commonly focused on the production of CBD and THC. [6] Since these cannabinoids are widely available from plants and generally not worth synthetic efforts, the competent total synthesis of minor cannabinoids still requires the expertise of a formally trained organic chemist to understand, choose, and adapt known methods to new targets. Organic chemists working towards the efficient, safe production of otherwise inaccessible phytocannabinoids seem now to have their work cut out for them, with many improvements in process efficiencies in development. Nevertheless, a variety of major and minor cannabinoids are feasibly produced at commercial scales using synthetic chemistry.
A Note on Chirality in Cannabinoids
Many cannabinoids are chiral, as are many natural products and derived medicines. ‘Chirality’, from the Greek for ‘hand’ (a familiar chiral object), refers to asymmetry in chemical bonding. Appropriately, the concept of chirality is often demonstrated using hands; both left and right hands are mirror images of each other, but one hand cannot be superimposed on the other. In a similar way, a chiral molecule has a non-superimposable mirror image, called an enantiomer, like CBD (Figure 4).
Enzymes in biological systems often exclusively produce only one of two possible enantiomers, resulting in the chiral purity and powerful bioactivity of natural products (i.e., plant-based medicines and poisons). Enzymes’ ability to construct chiral molecules has motivated chemists to develop synthetic methods to achieve similarly high chiral purity without enzymes. Such chiral synthetic methods are often developed in order to ‘recreate’ natural products at small scales to confirm molecular structures initially proposed using data from nuclear magnetic resonance or x-ray crystallography. If analysis of the synthetically produced compound matches that of the natural product, then the structure is considered known. This process of structural determination assisted by organic synthesis is how the structure of many plant metabolites and drug candidates are determined, and this is how the structure of (-)-trans-Δ9-THC was determined. [28] As a result, synthetic methods to produce nature-identical cannabinoids are well-established, demonstrating that a high degree of chiral purity can be achieved either botanically or synthetically. So, if nature-identical cannabinoids are produced synthetically, the expected bioactivity is only achieved if the chirality matches that of the natural product. It’s therefore a significant detail to consider, for producers, regulators and consumers alike, to verify the chiral purity (aka, ‘enantiomeric excess’) of cannabinoids produced outside of the plant. Such verification is readily achievable using chiral HPLC [29], although not currently a component of testing requirements for bringing minor cannabinoids to market.
The Future of Minor Cannabinoids
Much of what the future holds for the availability of minor cannabinoids depends on an evolving regulatory environment responsible for protecting the public health. The cannabis industries, both THC-dominant and hemp, have largely worked hand-in-glove with state and federal regulators to ensure the responsible growth of this new industry. However, with the rapid appearance of minors and cannabinoid derivatives, regulators have been caught off guard by the need to understand and oversee potential new public health hazards brought about by unspecified and largely unknown production practices that stray from the simple extraction of plant material. Some state regulators, without budget or experience overseeing what is more commonly FDA purview, upon learning of unspecified production of minor cannabinoids, whether semi- or fully-synthetic, have begun to ban the production of cannabinoids that are not directly extracted from cannabis plant material. And with FDA hamstringing its own regulatory authority by failing to approve any cannabinoid-containing product as a food or dietary supplement (i.e., such approvals would give FDA regulatory authority) states are left to devise independent oversight of cannabinoid production. This scenario is recipe for further complications such as the interstate commerce of hemp products, where one state’s laws may not guarantee products compliant with laws in another state. But a solution is at least conceivable. Since FDA already oversees the synthetic production of many natural products and derivatives (i.e., dietary supplements), the expanded availability of minor cannabinoids, regardless of production method, is achievable via existing regulatory structures. This can happen if the FDA would recognize hemp (or even better, cannabis as a whole) for what it is – an ingestible botanical product that, while not intended to treat disease as a pharmaceutical, contains ingredients to supplement the diet. This, in fact, aligns well with FDA’s definition of a dietary ingredient. Could it be simpler?
References
[1] Walsh KB, McKinney AE, Holmes AE. Minor cannabinoids: Biosynthesis, molecular pharmacology and potential therapeutic uses. Front Pharmacol. 2021;12:777804. [journal impact factor = 5.988; times cited = 6][2] Russo EB. The case for the entourage effect and conventional breeding of clinical cannabis: No “strain,” no gain. Front Plant Sci. 2019;9:1969. [journal impact factor = 6.627; times cited = 134]
[3] LaVigne JE, Hecksel R, Keresztes A. et al. Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci Rep. 2021;11:8232. [journal impact factor = 4.996; times cited = 16]
[4] Bachtel N, Israni-Winger K. Introduction: Plant-based medicine and pharmacology. Yale J Biol Med. 2020;93(2):227–8. [journal impact factor = 3.549; times cited = N/A]
[5] Radwan MM, ElSohly MA, El-Alfy AT, et al. Isolation and pharmacological evaluation of minor cannabinoids from high-potency Cannabis sativa. J Nat Prod. 2015;78(6):1271-1276. [journal impact factor = 4.803; times cited = 98]
[6] Sampson PB. Phytocannabinoid pharmacology: Medicinal properties of Cannabis sativa constituents aside from the “big two”. J Nat Prod. 2021;84(1):142-160. [journal impact factor = 4.803; times cited = 8]
[7] Nguyen GN, Jordan EN, Kayser O. Synthetic strategies for rare cannabinoids derived from Cannabis sativa. J Nat Prod. 2022 Jun 24;85(6):1555-1568. [journal impact factor = 4.803; times cited = 0]
[8] Pretzsch CM, Voinescu B, Lythgoe D, et al. Effects of cannabidivarin (CBDV) on brain excitation and inhibition systems in adults with and without Autism Spectrum Disorder (ASD): a single dose trial during magnetic resonance spectroscopy. Transl Psychiatry. 2019;9(1):313. [journal impact factor = 7.989; times cited = 19]
[9] Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5(11):1131-1141. [journal impact factor = 5.780; times cited = 251]
[10] Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199-215. [journal impact factor = ; times cited = 598]
[11] Rhee MH, Vogel Z, Barg J, et al. Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase. J Med Chem. 1997;40(20):3228-3233. [journal impact factor = ; times cited = 152]
[12] Tagen M, Klumpers LE. Review of delta-8-tetrahydrocannabinol (Δ8 -THC): Comparative pharmacology with Δ9 -THC. Br J Pharmacol. 2022 Aug;179(15):3915-3933. [journal impact factor = 9.473; times cited = 3]
[13] Anderson L, Heblinski M , Arnold J, et al. Cannabigerolic acid, a major biosynthetic precursor molecule in cannabis, exhibits divergent effects on seizures in mouse models of epilepsy. Br J Pharmacol. 2021 Dec; 178(24):4826-4841. [journal impact factor = 9.473; times cited = 11]
[14] Harvey DJ, Brown NK. In vitro metabolism of the equatorial C11-methyl isomer of hexahydrocannabinol in several mammalian species. Drug Metab Dispos. 1991;19(3):714-716. [journal impact factor = 3.384; times cited = 1]
[15] Thomas A, Stevenson LA, Pertwee RG, et al. Evidence that the plant cannabinoid Delta9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol. 2005 Dec;146(7):917-26. [journal impact factor = 9.473; times cited = 152]
[16] (a) Bueno J, Greenbaum, E. (−)-trans-Δ9-Tetrahydrocannabiphorol content of Cannabis sativa inflorescence from various chemotypes. J. Nat. Prod. 2021;84(2):531-536. [journal impact factor = 4.803; times cited = 5]
[17] Linciano P, Citti C, Luongo L, et al. Isolation of a high-affinity cannabinoid for the human CB1 receptor from a medicinal Cannabis sativa variety: Δ9-Tetrahydrocannabutol, the butyl homologue of Δ9-tetrahydrocannabinol. J Nat Prod. 2020;83(1):88-98. [journal impact factor = 4.803; times cited = 27]
[18] Brown NK, Harvey DJ. Metabolism of n-hexyl-homologues of delta-8-tetrahydrocannabinol and delta-9-tetrahydrocannabinol in the mouse. Eur J Drug Metab Pharmacokinet. 1988;13(3):165-176. [journal impact factor = 2.46; times cited = 2]
[19] Salbini M, Quarta A, Russo F, et al. Oxidative stress and multi-organel damage induced by two novel phytocannabinoids, CBDB and CBDP, in Breast Cancer Cells. Molecules. 2021;26(18):5576. [journal impact factor = 4.927; times cited = 0]
[20] Linciano P, Citti C, Russo F, et al. Identification of a new cannabidiol n-hexyl homolog in a medicinal cannabis variety with an antinociceptive activity in mice: cannabidihexol. Sci Rep. 2020;10(1):22019. [journal impact factor = 4.996; times cited = 17]
[21] Luo X, Reiter MA, d’Espaux L, et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature. 2019;567:123–126. [journal impact factor = 69.5; times cited = 290]
[22] Kjeldsen T, Balschmidt P, Diers I, Hach M, Kaarsholm NC, Ludvigsen S. Expression of insulin in yeast: the importance of molecular adaptation for secretion and conversion. Biotechnol Genet Eng Rev. 2001;18:89-121. [journal impact factor = 3.80; times cited = 25]
[23] Favero GR, de Melo Pereira GV, de Carvalho JC, de Carvalho Neto DP, Soccol CR. Converting sugars into cannabinoids—The state-of-the-art of heterologous production in microorganisms. Fermentation. 2022; 8(2):84. [journal impact factor = 4.97; times cited = 1]
[24] Tahir MN, Shahbazi F, Rondeau-Gagné S, Trant JF. The biosynthesis of the cannabinoids. J Cannabis Res. 2021;3(1):7. [journal impact factor = 4.786; times cited = 12]
[25] Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93(1):217-224. [journal impact factor = 16.38; times cited = 320]
[26] Appendino G. The early history of cannabinoid research. Rend. Fis. Acc. Lincei. 2020;31:919–929. [journal impact factor = 1.74; times cited = 11]
[27] Pollastro F, Caprioglio D, Marotta P, et al. Iodine-promoted aromatization of p-menthane-type phytocannabinoids. J Nat Prod. 2018;81(3):630-633. [journal impact factor = 4.803; times cited = 8]
[28] Nicolaou KC. The art and science of constructing the molecules of nature. Proc Natl Acad Sci USA. 2004;101(33):11928. [journal impact factor = 12.78; times cited = 6]
[29] Filer CN. Chirality in Cannabinoid Research. Cannabis Cannabinoid Res. 2021;6(1):1-4. [journal impact factor = 4.786; times cited = 1]