When it comes to the release of new medications, one of the most important aspects of the research and development process is the establishment of a safety profile. This is a collection of data regarding standard values that have become expected for pharmaceutical companies to provide with regards to their new product. Among other things, this data allows physicians and scientists to objectively assess the medication on their own, determine the appropriate dosing for different types of patients, and compare drugs in a meaningful manner. The safety profile is derived from a series of tests called Pharmacodynamics and Pharmacokinetics, which are done as part of the greater clinical trials spectrum.
Pharmacokinetics (PK) quantify where, at any given point in time, we can find the drug in question from the moment it’s been ingested to the moment it’s gone. Those physicians and scientists also like to find out what routes it chooses to leave from: sweat, urine, feces, etc. Summarized by the neat acronym ADME (Absorption, Distribution, Metabolism, and Excretion), PK also tells us how much of the compound reaches the target tissue, versus how much penetrates other organs or remains in blood circulation. In the case of cannabinoids, caffeine, and nicotine, this may be the central nervous system, the gastrointestinal tract, nerve endings, or cutaneous vasculature. [1]
These parameters are separate from Bioactivity, determined by the branch called Pharmacodynamics, which tells us how much a targeted receptor or signaling system is influenced in the presence of the drug that’s there. This helps determine the effective dose of the drug required. [2] This data helps inform physicians as to how much of the medication they ought to prescribe, at what frequency to recommend the patient take it, for how long, and if there are any side effects or contraindications (bad mixtures) to be aware of.
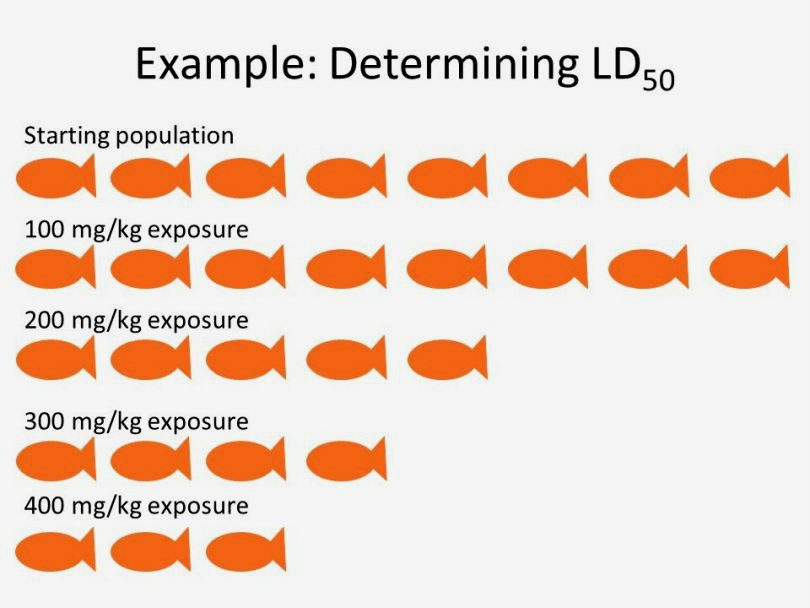
Successful clinical trials should demonstrate significant effectiveness of their treatment with minimal to moderate risk to health and safety. To that end, one of the most important values generated by these experiments is the LD50, or LD50. This is an experimentally-derived constant which signifies the dose or concentration at which 50% of animal subjects die from exposure. These numbers can be accessed via any scientific reference guide, online or in print, and are also available in OSHA-mandated Safety Data Sheets in laboratories or manufacturing facilities. Below we have compiled the LD50 for some common compounds we encounter in the cannabis world and in our everyday lives:
| Compound Name, Oral Ingestion: | LD50 (in units of mg of compound per kg of body weight, except where noted) |
| Table Salt (NaCl) | 3,000-4,000 (Mice vs Rats) |
| Water | 90 mL/kg |
| Caffeine | 192 (Rats) |
| Nicotine | 6.5-13 (Dogs) [3] |
| THC – Cayman Chemicals MSDS | 482-666 (Mice vs. Rats) [4],
>10,000 in primates (i.e. no deaths in study) [5]. |
| Camphor – EU Food Safety | >5,000 (Rats) |
| Menthol – NIH ToxNet | 2,900-3,100 (Rats vs. Mice) |
As you can see cannabinoids and terpenes are among the safest compounds we have around us, much safer on a per kilogram basis than even water or caffeine. Tables like these represent the importance of the LD50, as it allows us to clearly state that illegalization based on safety concerns is an unfounded mandate not based on scientific results.
References
- Alsherbiny, M.A. and Li, C.G. “Medicinal Cannabis—Potential Drug Interactions”. 2019; 6: 1-12 [Times cited = 0, recently published; Journal impact factor = 2.133]
- Hartsel, Joshua A., et al. Nutraceuticals: Efficacy, Safety and Toxicity. Chapter 53 – Cannabis sativa and Hemp. Academic Press, 2016. Pages 735-754.
- Mayer, Bernd. “How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century”. Arch Toxicol. 2014; 88(1): 5–7 [Times cited = 165, Journal impact factor = 5.980]
- Rosenkrantz, Harris, et al. “Inhalation, parenteral and oral LD50 values of Δ9-tetrahydrocannabinol in Fischer rats”. Toxicol Appl Pharmacol. 1974; 28(1): 18-27 [Times cited = 34, Journal impact factor = 3.705]
- Thompson, G.R., et al. “Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys”. Toxicol Appl Pharmacol. 1973; 25(3): 363-72 [Times cited = 84, Journal impact factor = 3.705]








Aw, this was an extremely nice post. Spending some time and actual effort to generate a really good article… but what can I say… I procrastinate a lot and never seem to get anything done.